Introduction
We are exposed to radiation in our daily life without notice. Radiation can be from natural sources such as ultra-violet ray or man-made from human innovation. Radiation from x-ray machine is example of man-made source. Daily used equipment like micro wave oven and wireless communication devices also emit radiation.
Radiation is a form of energy wave on the move. It is an electromagnetic wave that consists of two components, electric and magnetic field, moving together through space at the speed of light. Radiation can be divided into two categories: ionising radiation with high ionising energy and non-ionising radiation that does not have sufficient energy to cause ionization in living matter. The categorisation of radiation is shown in fig. 1.
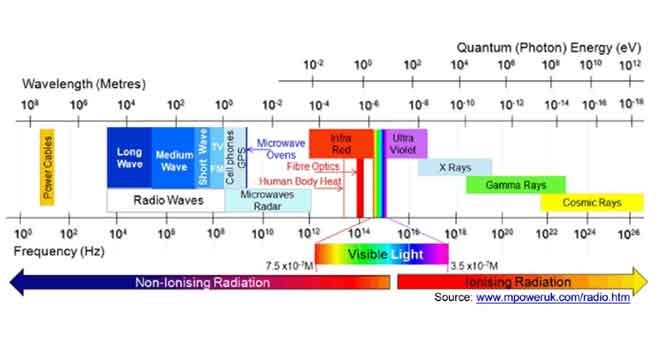
Fig 1: The Electromagnetic Spectrum
Scientific Officer (Physics)
Medical physics services in the Ministry of Health (MOH) has started since the establishment of Institute of Radiotherapy, Oncology and Nuclear Medicine in Kuala Lumpur Hospital in year 1968. Following the gazettement of Radioactive Substances Act 1968, In 1974, the scientific officer (physics) has been established at the Engineering Services Division to undertake the regulatory function.
The roles and functions of Scientific Officer (Physics) in the Ministry of Health are grouped into three main areas: regulatory control, clinical services and technical advisory as shown in Figigure 2.
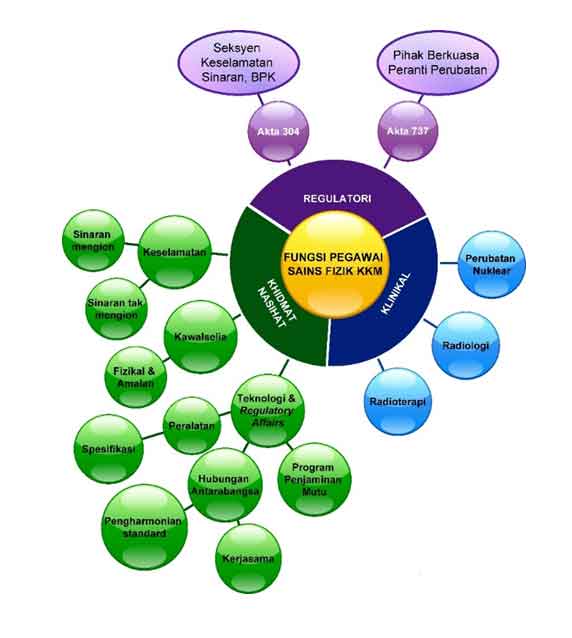
Fig. 2: Summary of Scientific Officer (physics) Roles in the Ministry of Health
At present, the Scientific Officer (Physics) are based at various offices and hospitals. Their roles and functions are as follows;
- Regulatory Control
- Radiation Safety Section, Engineering Services Division (enforcement of the Act 304)
- State Health Department (enforcement of the Act 304)
- Medical Device Authority (enforcement of the Act 737)
- Clinical services at MOH Hospitals
- Diagnostic Imaging Department
- Radiotherapy and Oncology Department
- Nuclear Medicine Department
- Technical Advisory
- Science Physics Unit, Engineering Services Division
- State Health Department
Regulatory Control
The use of ionising radiation in medical is regulated through enforcement of the Atomic Energy Licensing Act 1984 (Act 304) while other medical devices are subject to the Medical Device Act 2012 (Act 737) . Both acts, Act 304 and Act 737, are complimenting each other in handling radiation issues involving both ionising and non-ionising radiation. Provisions under Act 737 will be used in handling issues pertaining to medical devices that are not covered under Act 304.
-
Atomic Energy Licensing Act 1984 (Act 304)
This act is established for regulation and control of atomic energy and establishment of standards on liability for nuclear damage and related matters.
Section 15(2) of the act granted the Director-General of Health as ‘appropriate authority’ to issue separate licenses on behalf of the Board to persons applying for a license to undertake any activity in respect of medical purposes. Radiation Safety Section in Engineering Services Division has been given the task to undertake this activities on behalf of the Director-General of Health. This act however is limited to the device that utilizes ionizing radiation. -
Medical Devices Act 2012 (Act 737)
Medical Device Act 2012 (Act 737) is established to regulate medical devices, related industries and related matters. This act gives both authority and responsiblities to the Medical Device Authority through Medical Device Authority Act 2012 (Act 738) pertaining to medical devices control and regulation.
Act 737 has been enforced since 2013. According to the act, all medical devices to be marketed in Malaysia should be registered and marketed by licensed establishment. Medical devices authority officers consist of multidiscipline including Scientific Officer (Physics).
Clinical Services at Ministry ofHealth Hospitals
Medical physics services in hospital are closely related to medical procedures involving ionizing radiation, security of radioactive materials, radiation safety and quality assurance of irradiating equipment. The main role of Scientific Officer (physics) in MOH hospitals is to ensure radiation dose given to the patients are safe and effective in achieving intended diagnostic or therapeutic purpose as prescribed by the medical officer.
- Radiotherapy and Oncology
- Focus on patient treatment/ therapy
- To ensure treatment and radiation therapy received by the patients are as prescribed and planned as well as adhere to good scientific practice.
- Nuclear Medicine and Radiology
- Focus on diagnosis
- To ensure that all radiation equipment produce quality radiation with minimum exposure in accordance to the ALARA principal.
In clinical services, Scientific Officer (Physics) working closely with medical officer, biomedical engineer, radiographer, technologist and nurses. They also provide supervision of radiation safety of workers. Scientific Officer (Physics) also irradiating and related equipment are, maintained and calibrated, radiation produced are accurate, and functioning at their optimal levels.
Technical Advisory
Scientific Officer (Physics) also involve in providing technical advisory related to radiation issues (both ionizing and non-ionizing radiation) that may cause health effect. For example, issues on telecommunication base station, laser and electrical transmission line. Apart from that, they also coordinate and organise awareness programme related to these issues. Technical advisory function is carried out by the Scientific Officer (Physics) at Science Physics Unit in Engineering Services Division.as well as Scientific Officer (Physics) at State Health Department.
Among others, their function with regards to the technical advisory are as follows:
- Radiation safety
- Inquiry and complain
- Awareness training
- Radiation safety programme
- Security of radioactive material
- To develop programme that encompass physical protection and security culture
- To carry out security assessment
- Technology & Regulatory Affairs
- Device & technology
- Policy & legislation
- Standards harmonisation
- Interagency collaboration (national & international)
- Regulatory review
Summary
Roles and functions of Scientific Officer (Physics) in the Ministry of Health in handling radiation issues and medical device are carried out by multiple MOH agency according to mandate and jurisdiction stipulated in Act 304 and Act 737. Their rolse and function are summarized as below;
- Scientific Officer (Physics) working at the licensing and enforcement agency is responsible to ensure all regulatory requirement are in accordance to the Atomic Energy Licensing Act 1984 and Medical Device Act 2012.
- Scientific Officer (Physics) working in clinical services will involve issues related to scientific, effective, safety, security and efficient use of radiation;
- At policy level in MOH, their functions are more in ensuring the regulatory system are up to date with current development and country need as well as current international practices.
- Scientific Officer (physics) at State Health Department has to coordinate the implementation of radiation protection programme at state level.
References
- International Atomic Energy Agency, Role and responsibilities, education and training requirements for clinically qualified medical physicist, Human Health Services Series No. 25, IAEA, Vienna (2013)
- Laws of Malaysia, Act 304, Atomic Energy Licensing Act 1984
- Laws of Malaysia, Radiation Protection (Licensing) Regulations 1986, P.U. [A] 149/86
- Calcagini G, Censi F, Bartolini P, Electromagnetic immunity of medical devices: the European regulatory framework, Ann Ist Super Sanita 2007;Vol. 43, No. 3, p. 268-276
- Caruana CJ, Padovani R, Christofides S, Physics and Society: The medical physics profession and its contribution to healthcare
- Laws of Malaysia, Act 737, Medical Devices Act 2012
- https://radia.moh.gov.my/project/new/radia/perundangan.php
- http://www.mdb.gov.my/mdb/
- Smith PHS, The role of the medical physicist in relation to medical devices, IOMP
- Federal Government Gazette, Medical Device Regulation 2012, P.U. [A] 500
- Laws of Malaysia, Act 738, Medical Devices Authority Act 2012
| Last Reviewed | : | 04 March 2016 |
| Writer/Translator | : | Mohd Amin bin Yaakob |
| Accreditor | : | Dr. Bidi bin Ab. Hamid |







