Introduction
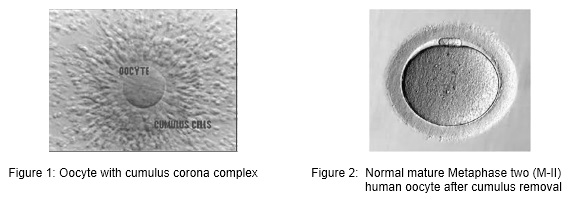
All over the world, millions of oocytes are harvested each year and culture in IVF laboratories with the aim of offering infertile couples the help of assisted reproductive technologies (ART). The human oocyte has a dimension of 120-160 µm and is the largest cell in human body. In many cases of ART, multiple oocytes are retrieved following different types of ovarian stimulation protocol. After oocyte retrieval procedure, the oocyte displays various aspects of maturation, integrity and viability. Direct assessment of the exact stage of nuclear maturation of the oocyte and its cytoplasmic characteristics are the two most important parameters to check and is often difficult as female gametes are surrounded by the cumulus and corona cells at the time of collection. The morphological feature of each oocyte was evaluated with the aid of inverted microscope.
For conventional IVF, oocytes are exceptionally stripped of cumulus cells in order to avoid any chemical or mechanical injury before standard insemination. For ICSI, cumulus and corona cells are routinely remove to allow an accurate assessment of oocyte nuclear maturity and to make oocyte handling easier during the microinjection procedure.
Oocyte quality, which is dependent on oocyte maturity, also plays a major role in the development potential of the embryo. There are two components of maturity – nuclear maturity and cytoplasmic maturity. Both components must occur in a coordinated and well-synchronized manner. It is generally recognized that a high-quality oocyte must complete nuclear maturity (M-II oocyte) and should have a round-clear zona pellucida, a small perivitelline space containing a single-non fragmented normal-sized first polar body, and a pale, moderately granular cytoplasm with no vacuoles, and a smooth endoplasmic reticulum.
In human oocytes, many types of abnormal morphology have been observed both within the cytoplasm and outside of the cytoplasm. The oocytes abnormalities were commonly classified as:
i) Oocytes with intracytoplasmic abnormalities,
ii) Oocytes with extracytoplasmic abnormalities and
iii) Oocytes with abnormal size and shape
Oocytes with intracytoplasmic abnormalities
Abnormal MII oocytes with intracytoplasmic abnormalities such as:
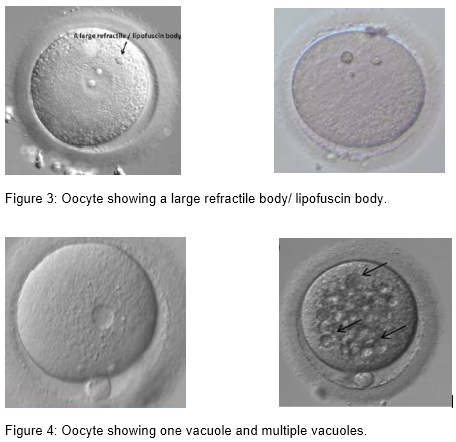
(i) Inclusion body/ refractile body and lipofuscin body
(ii) Presence of vacuoles
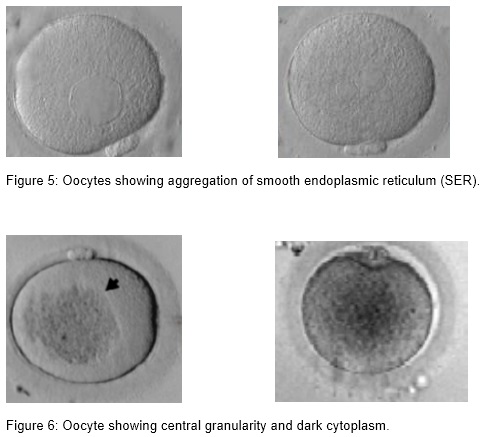
(iii) Presence of smooth endoplasmic reticulum (SER)
(iv) Granular and dark cytoplasm
Oocytes with extra cytoplasmic abnormalities
Abnormal MII oocytes with extra cytoplasmic abnormalities such as:
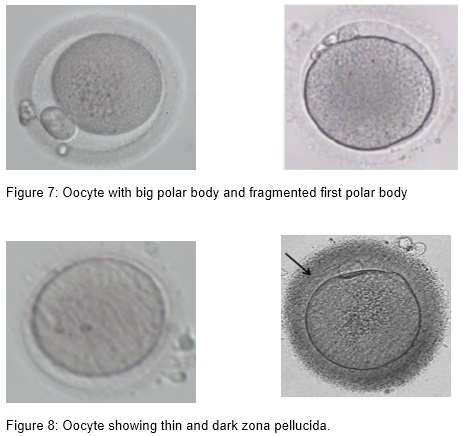
- Large polar body, fragmented polar body and multiple polar body
- Thin, dark, double, hairy brush-like zona pellucida,
- Wide perivitelline space
- Perivitelline debris
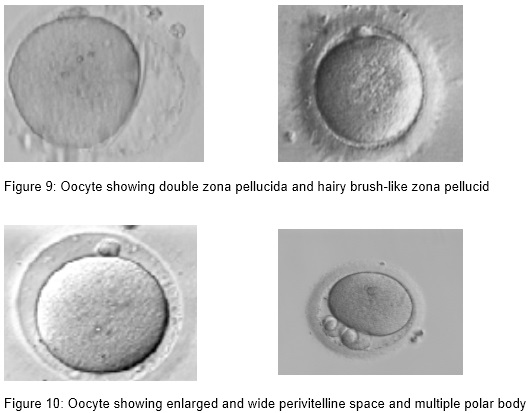
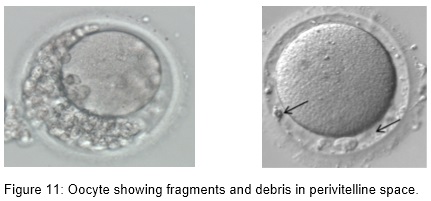
Oocytes with abnormal size and shape
Abnormal MII oocytes with abnormal size and shape such as:
- Giant oocyte
- Oval shape
- Crescent/ abnormal shaped cytoplasm
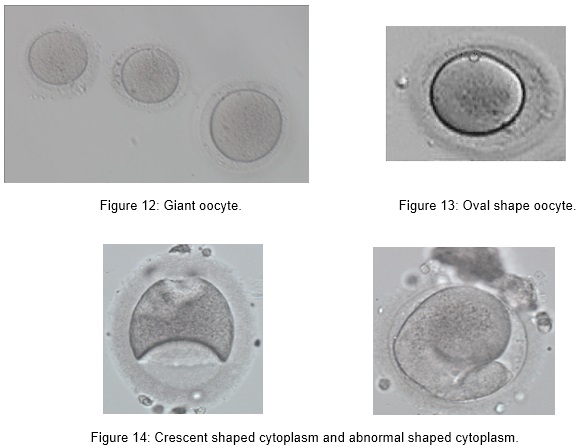
Implications of Oocyte Abnormalities
Oocyte quality is a limiting factor in female fertility. It is believed that the developmental fate of the embryo is largely determined by the quality of the oocyte. Aspects that may influence oocyte quality can be subdivided into morphological, cellular, and molecular. In routine IVF practice, the quality of oocytes is mostly determined by its appearance and its performance after fertilization. The main determinants for oocyte developmental competence are nuclear maturity, appearance of the spindle, size and cytoplasmic properties of the oocyte. Severe cytoplasmic deviations of the oocyte such as lipofuscin bodies, vacuoles, aggregation of smooth endoplasmic reticulum, central granularity, spindle aberrations, and especially the combination of several defects, do impair the developmental and implantation potential of the embryo. However, extracytoplasmic aberrations do not affect embryo development and implantation potential (Basak et al., 1998). Oocyte morphology can be included as a marker for predicting embryo quality and implantation potential additionally to standard morphological embryo scoring.
Lipofuscin/ refractile inclusions consist of a mixture of lipids and dense granular materials. They can be found in different stages of oocyte maturity. Only large lipofuscin inclusions, above 5 µm, are associated with significantly reduced fertilization and unfavorable blastocyst development (Otsuki et al. 2007).
Vacuoles are membrane surrounded cytoplasmic inclusions filled with fluid that is virtually identical to perivitelline fluid. Vacuoles may arise spontaneously during oocyte maturation when the first polar body is extruded (Van Blerkom, 1990), from pre-existing vesicles derived from the endoplasm ic reticulum or Golgi apparatus (El Shafie et al., 2000), or they are artificially created after ICSI (Ebner et al., 2005). Vacuoles appear in 4 % of the oocytes and have a negative effect on fertilization.The fertilization rate is negatively correlated with the number and size of the vacuoles; with a cut off value of 14 µm. Vacuoles also have a negative effect on blastocyst formation (de Sutter et al., 1996; Ebner et al., 2005).
Aggregation of smooth endoplasmic reticulum ( sER) is observed only in MII oocytes and has an incidence of 2 %. In cycles with affected oocytes the frequency can be as high as 25 %. Formation of these structures might correlate with high estradiol levels (Otsuki et al., 2004). The fertilization rate of the affected oocytes is not impaired if care is taken not to break the aggregation. sER aggregation is associated with an impaired blastocyst formation (18 %), a lower pregnancy outcome, obstetric problems, and a lower birth weight (Ebner et al., 2008, Otsuki et al., 2004).
Central granularity is a morphological feature of the oocyte that is recognized as a large, dark, spongy granular area in the cytoplasm. This anomaly represents a poor cytoplasmic maturity and is seen in 35% of the oocytes. Granularity has no effect on fertilization rate and embryo development or quality, but it is correlated with an impaired ongoing pregnancy rate of only 13 %. Preimplantation genetic diagnosis showed an aneuploidy rate of 52 % in blastomeres originating from these oocytes which might explain the high abortion rate (Kahraman et al., 2000).
First polar body (PB) morphology assessment in terms of fragmentation can be used to determine the postovulatory age of the oocyte. Fragmented polar bodies compared to intact polar bodies were believed to be associated with a lower embryo quality and lower fertilization, blastulation, implantation, and pregnancy rates (Ebner et al., 2002). However, because the first polar body has a very short lifetime, its morphology changes after a few hours of in vitro culture. Therefore, polar body morphology assessment may not serve as a reliable marker of oocyte quality and developmental competence (Verlinsky et al., 2003)
Irregularities in human zona pellucida (ZP) morphology sometimes will be noticed. ZP dysmorphology has an incidence of 2-5% of all oocytes. The ZP has an important role in oocyte fertilization and when thickening is present it may prevent implantation. It is essential for sperm binding and preventing polyspermy. It may have a protective role prior to hatching and protecting embryos from mechanical stress prior to implantation. However, some study shows that extracytoplasmic abnormalities of the oocyte do not affect fertilization, embryo development rate or implantation potential (Loutradis et al., 1999).
Perivitelline space abnormalities are among the most important dysmorphisms of the extracytoplasmic component. It has been reported that a large perivitelline space may be associated with increased oocyte degeneration and lower fertilization rates. However, studies have failed to find a correlation between the size and shape of the perivitelline space and fertilization rate or embryo development.
Oocytes normally have a diameter of 155 µm. Very small proportion (0.3 %) of the retrieved oocytes show a diameter that is approximately 30 % larger than normal oocytes (around 200 µm). These are called giant oocytes. Unfertilized giant oocytes appear to be diploid. Consequently, upon fertilization giant oocytes most frequently display three pronuclei, a phenomenon known as digynic triploidy. Resulting zygotes are capable of normal cleavage and development to the blastocyst stage. Because
they are chromosomally abnormal, it is not advised to transfer them, especially in order to avoid miscarriages (Rosenbusch et al., 2002).
On the other hand, the cellular and molecular aspects that may determine oocyte quality are not yet used in daily routine. They have not yet been fully examined, but they form an interesting and at the moment popular research topic. At present, cellular and molecular predictors of oocyte quality are intensively studied as they are believed to be more precise and objective indicators of oocyte competence and embryo quality compared to morphological parameters.
Subjects that are examined with great interest include:
- Comprehensive analysis of gene expression profiles of oocytes, and their associated cumulus cells by using microarray technology,
- Genetic constitution and cytogenetic analysis of polar bodies using comparative genomic hybridization ,
- Analysis of excreted proteins using mass spectrometry (proteomics) and
- Metabolomic investigations for indicators of oocyte quality in follicle fluids and culture medium.
More specific investigations include the mitochondrial status and glucose-6- phosphate dehydrogenase l activity in oocytes, apoptosis of follicular cells and levels of the transforming growth factor-? superfamily in follicular fluid (Revelli et al., 2009). Because most of these tests can be carried out non-invasively, these new approaches are expected to be ideal methods for identifying oocytes destined to produce chromosomally normal and viable embryos with high likelihood for live birth, thus improving the overall efficiency of the IVF process. However, all these investigations are costly, thus are not applicable to all the IVF couples.
Reference:
- Basak B, Bulent U, Aycan S,Cengiz A, Senai A, Ramazan M. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Human Reprod, 1998; vol 13; 12 3431 – 3433.
- De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. HumReprod. 1996;11:595-597.
- Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. RBMOnline 2008;16:801-807.
- Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, Tews G. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005; 83:1635 – 1640.
- Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod. 2002,17:2415-2418.
- El Shafi e M, Sousa M, Windt ML, Kruger T. An Atlas of the Ultrastructure of Human Oocytes. A Guide for Assisted Reprouction. Parthenon, NY. 2000.
- https://www.google.com/search.
- Kahraman S, Yakin K, Donmez E, Samli H, Bahce M, Cengiz G, Sertyel S, Samli M, Imirzalioglu N. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000; 15:2390-2393.
- Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999:72:240-244.
- Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007; 24; 263-2670.
- Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Human Reprod, 2004; 19; 1591-1597.
- Otsuki J. Intracytoplasmic morphological abnormalities in human oocytes. J. Mamm. Ova Res. 2009: 26: 26-31.
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009; 7:40.
- Rosenbusch B, Schneider M, Glaser B, Brucker C. Cytogenetic analysis of giant oocytes and zygotes to assess their relevance for the development of digynic triploidy. Hum Reprod. 2002;17:2388-2393.
- Sauerbrun-Cutler M, Mario V, Andrzej B, Eric G, Daniel S, Mathew L, Martin K. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. Journal of Ovarian Research (2015) 8:5 DOI 10.1186/s13048-014-0111-5
- Savitha P, Ramesh Raja D, Pandiyan R. Normal and abnormal oocytes observed during assisted reproductive technique (ART) procedures. Chettinad Health City Medical Journal. 2012.
- Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990; 16: 324-346.
- Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, Kuliev A. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? RMBOnline 2003; 7:336-341.
| Last Reviewed | : | 20 May 2016 |
| Writer | : | Tang Hoay Loon |
| Accreditor | : | Krishnan A/L Kanniah |







