Introduction
All medications either pharmaceuticals or traditional medicines must first be registered with the Drug Control Authority (DCA), Ministry of Health Malaysia before it can be imported, manufactured or sold in Malaysia. The objective of product registration is to ensure that all registered pharmaceutical products have been evaluated in terms of safety, efficacy and quality, while the traditional products evaluated and tested for safety and quality with the main aim of protecting and preserving the people’s health.
Benefits
Various measures have been taken in order to ensure that all products which can be accessed and used by the public are safe and of quality.Controlled medicines containing schedule poisons and general medicines (Over The Counter Medicines) are ensured safe, of quality and effective as it intended for whereas traditional medicines are ensured of quality and safe where the level of microbial content and heavy metals such as lead, Arsenik, mercury and Cadmium are at acceptable level.Before a product is approved to be marketed in Malaysia, it must first go through the various stages of evaluation and testing based on the principles of quality, efficacy and safety by the National Pharmaceutical Regulatory Agency (NPRA) as the secretariat to the DCA. In addition, the NPRA also monitors products that have been registered through surveillance and pharmacovigilance activities (the monitoring of adverse drug reactions) to ensure that the quality, safety and effectiveness of medicines marketed in Malaysia are maintained as well as in compliance to the standards set by the DCA.
Differences between Registered & Unregistered Medicines
A registered medicine is a medicine which has been approved by the DCA to be sold or used by consumers in Malaysia. These medicines have been evaluated, tested and proven to be of quality, effective and safe.
On the other hand, an unregistered medicine has not gone through the processes of evaluation and testing as specified above. Thus, the safety for use of such medicines is not guaranteed. In addition, unregistered medicines may also contain prohibited substances that can endanger the health of consumers and cause serious side effects if used over a long period of time.
Tips for Consumers
-
Get your medication from reliable sources such as pharmacies, clinics and hospitals.
-
Check the authenticity of the product registration number and Meditag hologram label on the product.
-
Check the label before buying or using any medicines.
-
Make sure that all medicines used have been registered with the DCA Beware of health products that have excessive claims such as diabetes, stroke and so forth.
-
Seek advice and further information on medicines from your doctor or pharmacist.
How to Check the Product Registration Status
Consumers can check whether a product is registered with the DCA via the Registered Products Search service available on the NPRA website (www.npra.gov.my) as shown in the diagram below. Review of this database can be done using either the product name, product registration number, the name of the product registration holder, the name of the active ingredient or the name of the product manufacturer.
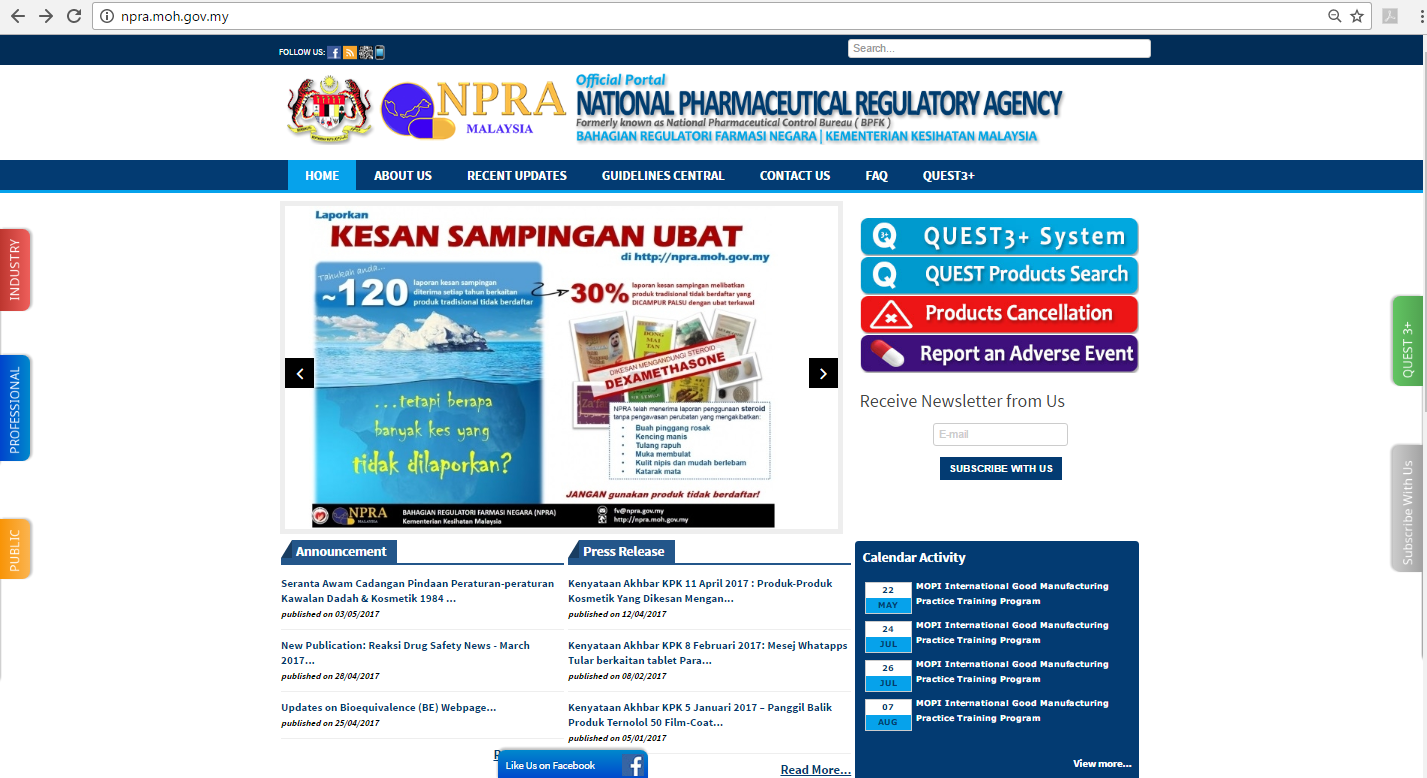
Visit the website npra.moh.my

Click on QUEST3 menu and select products search

Check the database by using either the name of the product, the product registration number, name of product registration holder, the name of the active ingredient or the name of the manufacturer of the product
Checking the Status of Products Containing Prohibited Substances
Consumers can browse the website www.pharmacy.gov.my to find out about verify on the registered products as well as unregistered products that have been identified to contain prohibited substances.
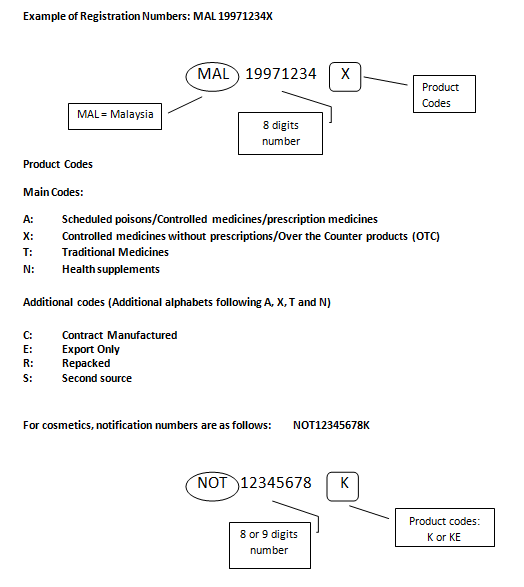
Product Registration Number (‘MAL’ Number)
Medicines that have been registered will be given a registration number that must be printed on the label and packaging of the medicines. This registration number starts with MAL (an acronym for Malaysia), followed by eight digits, and ends with an alphabet(s)/character(s) that reflects the category of the product. An example of a product registration number is as described below:-
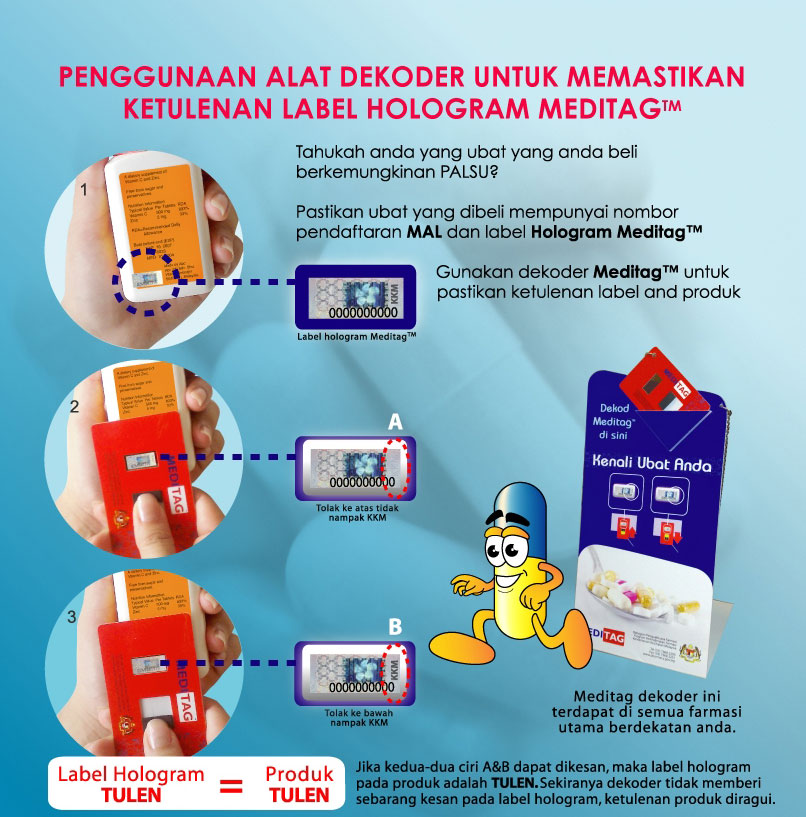
Hologram Label
Aside from the registration number printed on each registered medicinal product, the Ministry of Health Malaysia (MOH) has also made the use of the MeditagTM Hologram sticker labels mandatory as of 1st May 2005 to facilitate the identification of registered medicines. This measure was yet another proactive effort taken by the ministry to enhance the product identification and safety as well as to trace and eradicate the presence of fake medicines, counterfeit and unregistered products. Consumers can authenticate the hologram stickers for products purchased by using a decoder that has been supplied and is available at major pharmacies throughout the country. This device is placed on the counter of such premises for easy use by the public to verify the authenticity of products purchased as shown in the diagram below:
| Last Reviewed | : | 23 April 2014 |
| Writer | : | Azlina bt. Ismail |
| Accreditor | : | Norhayati bt. Musa |
| Reviewer | : | Mazlan bin Ismail Siti Nurul Fathihah bt. Baharudin |







