Introduction
Embryo cryopreservation or embryo banking is a method used to preserve embryos by cooling and storing them at low temperatures (-196oC) in liquid nitrogen. Embryos can be thawed at a future date and transferred to the uterus, providing additional opportunity for achieving conception.
History
The era of successful human embryo cryopreservation followed the discovery and development of chemical cryoprotectant solutions in 1949. In 1983, first pregnancy was reported for frozen embryo transfer.
Indication
The cryopreservation of human embryos is of great significance in assisted human conception. Not all the embryos developed in an in vitro fertilization (IVF) program can be transferred, owing to the risk of multiple pregnancies, thus necessitating the storage of the surplus embryos in liquid nitrogen. Cryopreservation provides further possibilities for conception and a cost effective alternative to fresh IVF cycles. Besides, cryopreservation also avoids a situation in which an embryo is transferred under adverse condition and allows the transfer of embryos with more flexible timing and endometrial preparation.
Method/ Procedure
Human embryos at different cleavage stages are usually frozen using a slow- freezing
protocol which means a slow, controlled rate of cooling through freezing planner, usually in the range of 0.1 – 0.3 oC/ min, after exposing the embryos stepwise to low concentrations of cryoprotectants, such as glycerol and propanediol, supplemented with sucrose.
A cryoprotectant is a chemical compound that limits damage to biological cells in freezing conditions. Without protection, cells will rupture when they freeze as a result of ice crystal formation.
During slow-cooling, cells dehydrate, shrink and the concentration of solutes increases as water freezes in the medium. As the surrounding temperature goes on decreasing, medium surrounding the cells will undergo physical and chemical changes. Liquid nitrogen will hold the embryos by achieving a temperature at -196 oC through a slow controlled rate of cooling process which takes approximately 2 hours.
Embryos will be loaded into a straw after exposed to cryoprotectant solutions and the straw will put into a small goblet which fixed tightly with a cane. All straw, goblet and cane are labeled with patient’s name and identity number. The frozen straw with cane will then put into a freezing cannister which is kept in the liquid nitrogen tank for storage.

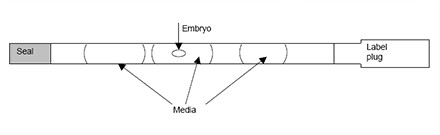
(a) Freezing planner (b) Embryo is loaded into a straw.



(c) Small goblet (d) Cane (e) Liquid nitrogen tank
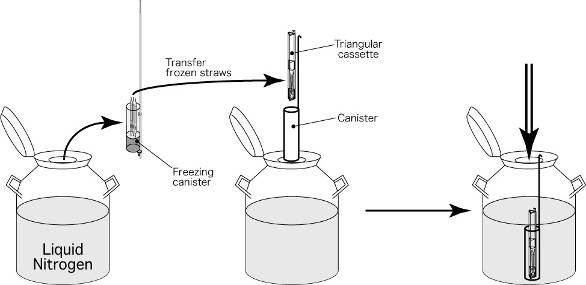
(f) Frozen Straws are transferred to a cannister and keep in liquid nitrogen tank at temperature -196 oC.
Current Status
Vitrification (rapid cooling) method has slowly replacing the conventional cryopreservation method. This is due to vitrification involves rapidly lowering the temperature of the embryos approximately 600 times faster than the conventional slow freeze method. The rapid drop in temperature enables the liquid containing the embryo to transition directly to a solid (glass-like) state thus preventing any ice formation.
Period of Storage
The embryos are stored in liquid nitrogen, where they can remain frozen for up to 10 years. However, patients are required to renew their consent for their embryo cryopreservation each 5 years for government facilities or each year for private centers.
Considerations and Risk
Optimal quality of the embryo is essential in order to guarantee its survival upon freezing. Frequently, low quality embryos are frozen which results in low survival rates. Cell damage does not occur in the storage phase if stored in the liquid phase of liquid nitrogen. It is the freezing and thawing process that is crucial to cell survival. Cross-contamination may happen in the same liquid nitrogen tank, thus screening test such as Hepatitis B, Hepatitis C, HIV and Syphilis tests need to be done in earlier stage before starting the IVF program.
Outcome
The implantation potential of cryopreserved, early-stage embryos is directly related to the blastomere survival rate. Therefore, only those embryos that are cleaving at acceptable rates and demonstrating little or no fragmentation (generally less than 25%) are normally cryopreserved. Embryos which are intact after thawing have the same implantation potential as fresh embryos and it is the loss of blastomeres which determines the decrease in implantation potential.
Reference
1) Gardner K: Embryo Cryopreservation Vitrolife GI Series TM Manual 2.0.7, 2003.
2) Mazur P, Principles of Cryobiology. In: Fuller BJ, Lane N, Benson EE, editors. Life in the frozen state. New York: CRC Press; 2004. p. 3-65.
3) Nagy ZP, Varghese AC, Agarwal A: Practical manual of in vitro fertilization. Springer: USA, 2012.
4) Prakash, T., Priti, P. T.: The heart and soul of ART …… is in the laboratory. Jaypee Brothers Medical Publishers: India, 168-205, 2004.
| Last Reviewed | : | 10 Mac 2015 |
| Writer | : | Helen Tang Hoay Loon |
| Translator | : | Helen Tang Hoay Loon |
| Accreditor | : | Krishnan a/l Kanniah |







