Introduction
Hydroquninone in cosmetic product is only allowed to be used in the preparation of artificial nails systems and restricted to professional use only. Hydroquinone or mixture with p-hydroxyanisole is functioned as a stabiliser in the preparation of artificial nails systems at maximum concentration of 0.02% after mixing. The use of such products containing hydroquinone should be avoided on the exposed skin and the warning should be clearly stated on the label.
Therefore other products containing Hyroquinone are regulated as pharmaceutical products and must be registered with the Drug Control Authority (DCA), Ministry of Health Malaysia (MOH) where it can only be used under the supervision of health professionals.
The misuse of Hydroquinone in Cosmetic Products
The use of hydroquinone is often associated with cosmetic product for skin whitening or to treat pigmentation problems. Hydroquinone is not allowed in cosmetic products thus these products are categorised as adulterated products. Adulterated products are against the stipulated guidelines and regulations.
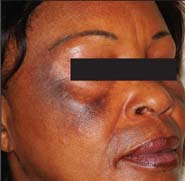
Unsupervised use of products containing hydroquinone can cause various adverse effects. Hydroquinone can cause redness on the skin, discomfort, skin discoloration or skin hypersensitivity. Hydroquinone can impede the process of pigmentation which reduces skin protection from harmful UV rays causing an increase risk of skin cancer. Long term use of such products may cause the skin to turn into black patches when exposed to sunlight. This is also known as exogenous ochronosis and the effects are irreversible.
Unsupervised use of products containing hydroquinone by health professional can cause various adverse effects. Hydroquinone can cause redness on the skin, discomfort, discoloration of the skin or skin becomes hypersensitive. Hydroquinone can impede the process of pigmentation and this process can reduce skin protection from harmful UV rays which may increase the risk of skin cancer. Long term use of such products may cause the skin to turn into black patches when exposed to sunlight. This is also known as exogenous ochronosis and the effects are irreversible.
The Roles of Consumer
Consumers are advised to get sufficient information before purchasing a cosmetic product and should not buy or use cosmetic products from dubious sources such the night market, street seller or online purchase. In addition, the consumer is also advised not to be easily lured by advertisements and any form of promotions that promoted extreme claims and promising positive effects instantenously.
Consumers and public may refer to NPRA official website at www.npra.gov.my to verify the notification status of cosmetic products or download NPRA Product Status Application from Google Play Store before purchasing such products. A list of cosmetic products tested and found to contain prohibited substances such as hydroquinone is published on the NPRA’s website.
Consumers are encouraged to report to the NPRA, if they experience any allergic reactions or adverse effects from the use of cosmetic products or encounter any suspicious cosmetics using Notified Cosmetic Complaint Form, available through the link:https://npra.gov.my/images/public/BORANG_ADUAN_KOSMETIK.pdf and e-mail to kosmetik@npra.gov.my for further action.
References
- Cosmetic Ingredient Review, Amended Safety Assessment of Hydroquinone as Used in Cosmetics http://www.cir-safety.org dilayari pada 23/6/2014
- Guidelines for Control of Cosmetics Products in Malaysia, Mei 2009 (rev06)
- Poison List accessed on 16/2/2015 at http://www.pharmacy.gov.my/
- Understanding Black Skin: Hyperpigmentation and Dark Marks. http://www.changemagazineonline.com/understanding-black-skin-hyperpigmentation-and-dark-marks/ dilayari pada 16/2/2015
- Cancellation of cosmetic products due to adulteration accessed on 16/2/2015 at www.npra.gov.my
| Last Reviewed | : | 22 August 2019 |
| Writer/Translator | : | Nurhirza bt. Shaiful Yamin |
| Accreditor | : | Noor Hidayah bt. Mohd Nor |
| Reviewer | : | Wan Mohaina bt. Wan Mohammad |
| : | Hanum Maisarah bt. Abdul Rahman |







