Introduction: Importance of Vaccination
Rotavirus is named because of its similarity in appearance to a wheel (rota means wheel in Latin) when viewed by electron microscopy. Rotavirus is the most common virus causing severe diarrhoea and vomiting that leads to severe body fluid loss among infants and young children throughout the world. Rotavirus disease is highly contagious and almost all children were infected by the age of 5. In Malaysia, about 22% to 50% cases of all diarrhoea in children are caused by rotavirus.
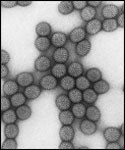
Figure 1: Electron micrograph of rotavirus
Rotavirus spreads easily from hand-to-mouth due to contact with stools from an infected person. Most of the children recover on their own from the infection. However, some children are hospitalised due to severe vomiting and diarrhoea with severe fluid loss that can be fatal. Fortunately, the infection can be prevented by vaccinating against the rotavirus.
Rotavirus vaccines are live vaccine and when given to an infant, the body’s natural defense will make antibodies against the rotavirus to prevent diarrhoea and vomiting caused by rotavirus. Currently there are 2 types of rotavirus vaccine available in the Malaysian market, series of 2 doses and 3 doses. Both are proven to be safe and efficacious in preventing severe diarrhoea in young children. According to data from clinical trials, Rotavirus vaccine was found to be effective in preventing 85% to 98% of severe rotavirus disease and 74% to 87% of all rotavirus disease.
World Health Organization (WHO) estimated that in 2008, nearly half a million of children die from rotavirus infection, accounted for about 5% of all child death. WHO recommends the rotavirus vaccine to be included in all national immunisation programmes especially in countries with high risk of severe disease and fatality rate due to rotavirus infection.
Indication and Target Group
Rotavirus vaccination is not included in our national immunisation programme. Rotavirus vaccine is indicated for infants 6 weeks and above for the prevention of diarrhoea and vomiting caused by rotavirus infection and may be given from age 6 weeks to 32 weeks depending on the type of vaccine given. However, the vaccine should not be given if:
- A baby has had a severe allergic reaction to rotavirus vaccine or allergic to any component of the vaccine.
- A baby with a rare disorder that affects the immune system called Severe Combined Immunodeficiency (SCID).
- A baby has had a type of bowel blockage called intussusception.
- A baby was born with malformation in the gut that could lead to bowel blockage.
The time of vaccination need to be postponed if the baby suffering from severe infection with high fever, diarrhoea and/or vomiting.
Dosage and administration
The table below shows the dosing for the different types of rotavirus vaccines:
|
Dose
|
Dosing interval
|
|---|---|
| Vaccine in series of 3 doses can be given up to age of 32 weeks | First dose administered at 6 to 12 weeks of age. Subsequent doses administered at minimum interval of 4 weeks between each dose. The vaccination course should be completed before 32 weeks of age. |
| Vaccine in series of 2 doses can be given up to age of 24 weeks | First dose administered from the 6 weeks of age. Subsequent dose administered at minimum interval of 4 weeks. The vaccination course should be completed before 24 weeks of age. |
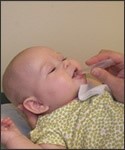
Figure 2: Rotavirus vaccine is given by mouth only
- Both vaccines are given by mouth.
- If the baby is given the vaccination series of 2 doses, in case if the baby spits out or regurgitates most of the vaccine dose, a replacement dose may be given at the same vaccination visit. However, do not repeat the dose if the baby spits out or regurgitates when he/she is administered with a 3-dose series vaccine. The baby should continue to receive any remaining doses of vaccine according to schedule.
- Rotavirus vaccine can be given to pre-term babies with the same vaccination course as full-term babies.
- Rotavirus vaccine can be given regardless of food, liquid or breast milk, either before or after vaccinated.
Possible side effects
Like all vaccines, there is a chance of side effects after administration of vaccine. The side effects are usually mild and temporary. The most common side effects include diarrhoea, vomiting and increased irritability.
However, there is also a small chance of serious allergic reaction with signs and symptoms such as rashes, swelling of the face and throat, difficulty in breathing, increased heartbeat, dizziness and weakness.
Very rarely, the babies experience bowel blockage (intussusception). Parents are advised to observe the baby if the baby develops signs and symptoms such as stomach pain with severe crying, persistent vomiting, blood in stools, a swollen belly and/or high fever.
Points to remember
Warning and Precaution
Like other vaccines, no vaccine is 100 percent effective and rotavirus vaccine may not completely protect all children who are vaccinated even after completion of the full course of vaccination. These vaccines will not provide protection against diarrhoea and vomiting caused by germs other than rotavirus.
Parents are advised to wash their hands thoroughly after changing their children’s nappies as the rotavirus vaccine virus is shed during the first weeks after vaccination.
Parents are advised to talk to a doctor or pharmacist before giving rotavirus vaccine to their child if:
- A baby has had received blood transfusion or blood product including immunoglobulin within 6 weeks.
- A baby has any chronic gastrointestinal disease.
- A baby has a close contact such as a household member who has a weakened immune system.
- A baby has growth problems.
- A baby has any disease or is on any medicines that may reduce his/her immune system against infection.
Rotavirus Vaccine and Other Vaccines
Rotavirus vaccine can be given together with other recommended vaccination such as diphtheria, tetanus, pertussis, Haemophilus influenzae type b, inactivated polio, hepatitis B, pneumococcal conjugate and meningococcus group C conjugate vaccines.
Storage Condition
Both vaccines must be stored at refrigerator temperature (2oC to 8oC), protected from light and must not be frozen.
Conclusion
Rotavirus vaccine is the best way to protect children against rotavirus infection. The vaccines are proven to be effective at preventing severe rotavirus disease in infants and young children. Besides a very low risk of bowel blockage (intussusception), the vaccines are considered safe and well tolerated. Although rotavirus vaccination is not part of our national immunisation schedule, parents are advised to consult a doctor for rotavirus vaccination to protect their children from rotavirus infection. Good personal hygiene practice such as hand washing is also important in controlling the spread of the rotavirus infection.
References
- Hsu, V.P., Abdul Rahman, H., Wong, S.L., Ibrahim, L., Yusoff, A.F., Chan, L.G., et al. (2005). Estimates of the burden of rotavirus disease in Malaysia. Journal of Infectious Diseases, 192 (Supplement 1), S80-S86.
- Poo, M.I. & Lee, W.S. (2007). Admission to hospital with childhood gastroenteritis in Kuala Lumpur, Malaysia. Medical Journal of Malaysia, 62(3), 189-193.
- World Health Organization (2013). Rotavirus vaccines. WHO position paper – January 2013. Weekly Epidemiological Record, 88, 49-64.
- Centers for Disease Control and Prevention (2012). Epidemiology and Prevention of Vaccine-Preventable Disease (12th ed.). Washington DC: Public Health Foundation.
- Palmer, E. (1978). [Rotavirus]. Retrieved February 25, 2015 from http://phil.cdc.gov/phil/details.asp?pid=197
- Rotavirus Vaccination (n.d.). Retrieved February 25, 2015 from http://www.cdc.gov/vaccines/vpd-vac/rotavirus/default.htm?s_cid=cs_074
| Last Reviewed | : | 13 November 2015 |
| Writer | : | Lim Bee Yee |
| Accreditor | : | Azura bt. Abdullah |







